Fibi IACUC – Institutional Animal Care and Use Committee Management System
Comprehensive Oversight for Ethical and Compliant Animal Research
Manage the complete lifecycle of animal research protocols in one system, ensuring transparency, regulatory compliance, and institutional accountability.
Why Research Institutions
Choose Fibi IACUC
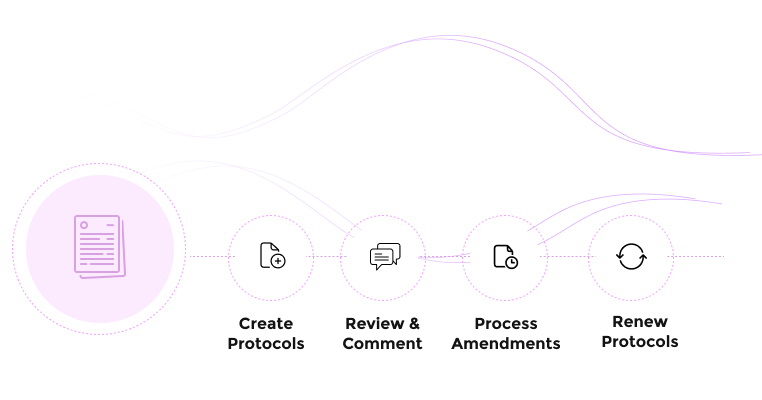
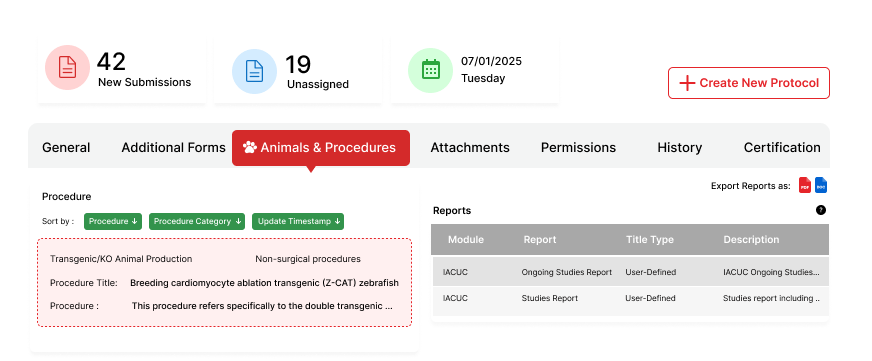
Complete Protocol Management
Manage the full lifecycle of animal research protocols within a single, integrated system.
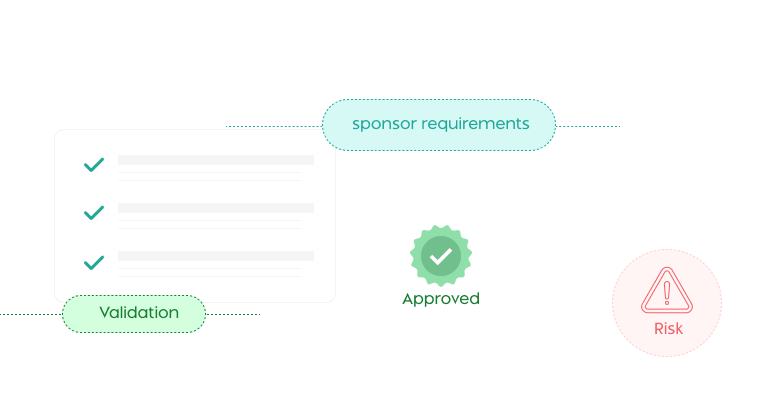
Humane Research Compliance
Ensure ethical treatment and regulatory conformity for laboratory animals
Detailed Procedure Tracking
Record species, procedures and materials, with precision and traceability.
Flexible Review Options
Support all review types from administrative to full committee
Role-Based Workflows
Customize processes for PIs, reviewers, and administrators
Amendment & Renewal Tracking
Easily manage amendments and track continuing reviews or renewals.
Key Features
Comprehensive Protocol Creation
Build detailed protocols with species-specific information.
Protocol Templates
Easily create new submissions based on existing protocols.

Procedure Documentation
Capture details such as procedure category, type, and pain/distress levels.
Species & Strain Tracking
Record species names, strains, counts, and USDA categories.

Digital Certification
Secure electronic certification with comprehensive audit trail.

Multiple Review Types
Support administrative, full committee, designated member, and veterinary reviews.

Interactive Review Interface
Add comments and attachments during the review process.
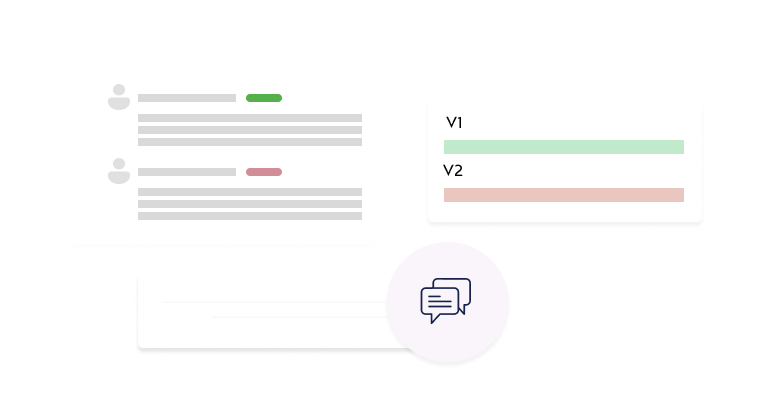
Protocol Comparison
Compare versions side-by-side to easily identify modifications.
Version History
Track all changes across submissions with complete version logging.

Renewal Management
Streamline annual reviews with automated notifications.

Procedure Supplements
Document materials used in procedures.
Location Tracking
Log housing and procedural locations.

Personnel Assignment
Link qualified staff to specific procedures and animals.
Veterinary Verification
Support for veterinary consultation and verification.

Animal Reports
Generate detailed reports on animals used in research.
Configurable Dashboards
Role-specific views for PIs, reviewers, and administrators.
Automated Notifications
Schedule reminders for renewals, expirations, and training requirements.
Meeting Management
Generate agendas and minutes for committee meetings.
Action Tracking
Comprehensive history of all protocol actions and decisions.

Customizable Forms
Capture institution-specific information through dynamic questionnaires.
Seamless System Integration
Connect seamlessly with Fibi’s research suite for end-to-end oversight.

Customizable Validations
Configure business rules for protocol validation.

Ad-hoc Communications
Send targeted notifications to relevant stakeholders.

Training Compliance
Monitor and validate training requirements for all assigned personnel.

Protocol Closure Management
Automated protocol closure processes.
How Fibi IACUC Transforms Animal Research Compliance
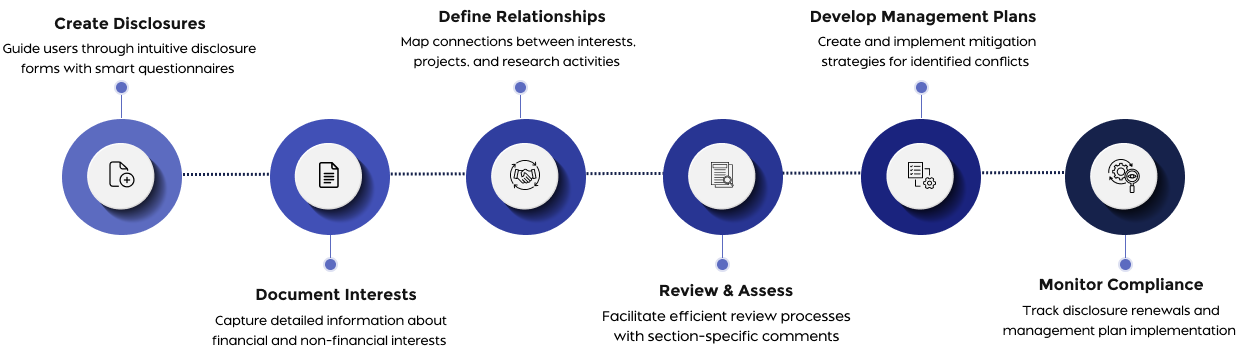
FAQs
What is Fibi IACUC and what does it do?
Fibi IACUC is an electronic management system for Institutional Animal Care & Use Committees. It oversees the entire lifecycle of animal research protocols in one platform, helping institutions maintain ethical, compliant animal research. Key functions include protocol development, review workflows, approval, and tracking of all animal use records.
How does Fibi support protocol compliance and tracking?
Does Fibi IACUC handle renewals, amendments, and multiple review types?
Can Fibi IACUC customize workflows by role?
Is Fibi IACUC integrated with the rest of Fibi’s suite?
Can Fibi IACUC be deployed on-premise or in the cloud?
What is the typical implementation timeline for Fibi IACUC?
What kind of training and support is included in the implementation process?
Can institutions migrate legacy data into Fibi during implementation?
The Complete
Fibi Research Management Suite
Fibi IACUC integrates seamlessly with the broader Fibi platform, unifying compliance, pre-award, and post-award activities across your institution:
Ready to Transform Your IACUC Process?
Experience how Fibi IACUC can revolutionize animal research compliance at your institution.





