IRB Management Software for Institutional Review Boards
Streamline Human Subject Research Compliance
Enhance IRB review process with a purpose-built electronic IRB Management system that supports transparency, efficiency, and regulatory compliance.
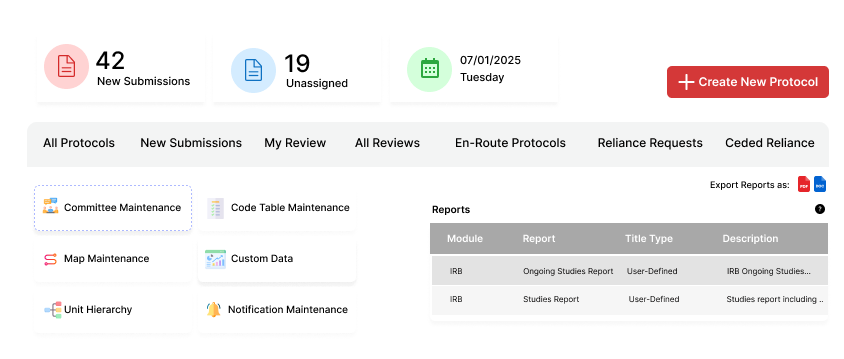
Why Research Institutions Trust Fibi IRB Compliance Software
Unified Protocol Management
Manage the entire IRB lifecycle with our advanced IRB compliance software, ensuring smooth research
Collaborative Environment
Connect principal investigators, IRB committees, reviewers, and administrators through a shared platform.
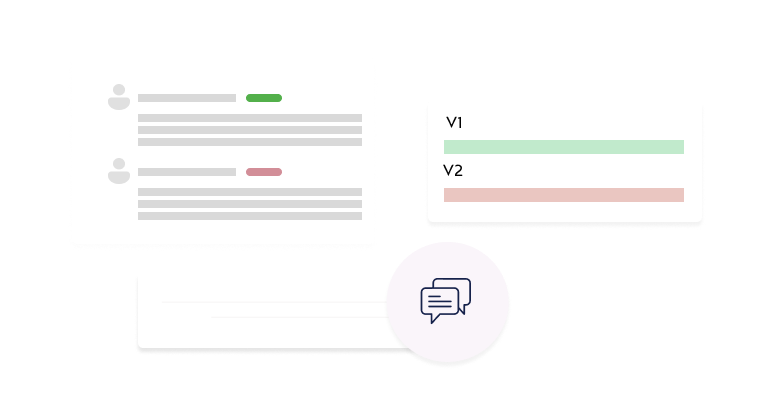
Interactive Review Tools
Enable efficient reviews with section-specific commenting, version comparisons, and decision tracking.
Meeting Management
Schedule IRB meetings, auto-generate agendas, capture minutes, and record decisions effortlessly
Configurable Workflows
Customize approval processes to match institution's needs
Seamless Integration
Link IRB functions with Fibi’s pre-award and post-award modules for end-to-end research management.
Automated Compliance
Ensure adherence to regulatory requirements with built-in reminders and notifications
Key Features
Protocol Lifecycle Management
- Collaborative Protocol Development: Build applications with an intuitive, section-based interface designed to support research ethics review and IRB workflows.
- Version Control: Track changes across submissions with comprehensive version history.
- Protocol Comparison: Compare versions side-by-side to easily identify modifications.
- Digital Certification: Secure electronic certification with complete audit trail.
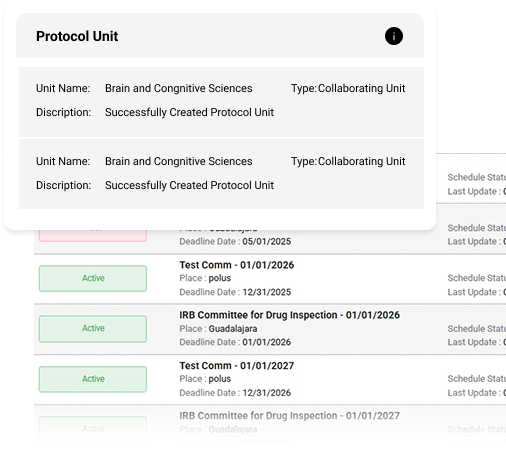
Review Management
- Interactive Review Interface: Add section-specific comments with public or private visibility.
- Single-Page Protocol View: Review complete protocols in a consolidated, easy-to-navigate format.
- Comprehensive Commenting System: Enable direct communication between reviewers and investigators.
- Committee Management: Centralize committee membership, scheduling, and documentation.
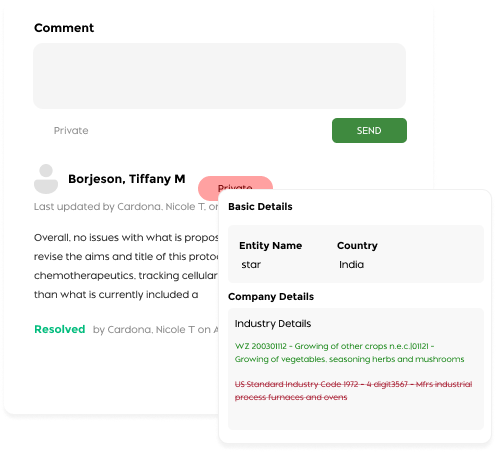
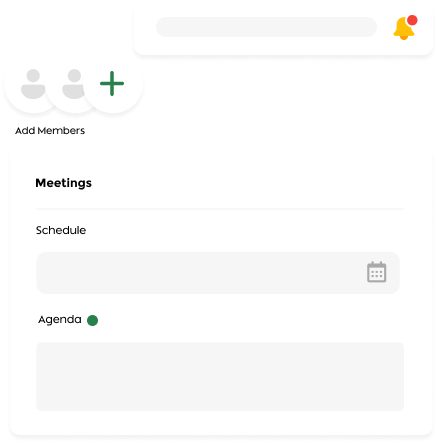
Meeting Lifecycle Management
- Automated Agenda Creation: Generate and distribute meeting agendas from configurable templates.
- Minutes Management: Create, review, and distribute meeting minutes efficiently.
- Quorum Tracking: Monitor attendance requirements in real time to ensure compliance.
- Scheduled and Ad-hoc Meetings: Automatically generate meeting schedules.
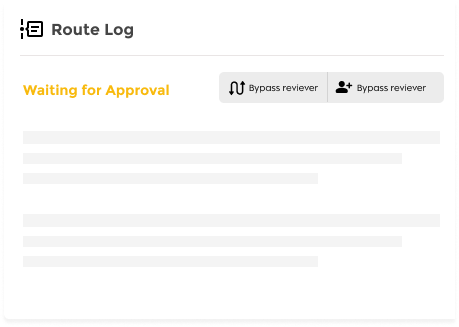
Workflow Lifecycle Management
- Configurable Routing: Customize approval paths based on institutional requirements
- Role-Based Actions: Assign specific actions to PIs, administrators, reviewers, and committees
- Automated Notifications: Trigger alerts for renewals, expirations, and pending reviews.
- Amendment & Renewal Processing: Streamline modification and continuation requests
Reporting & Communication
- Configurable Correspondence: Generate automated communications using configurable templates
- Dashboard Analytics: Monitor key metrics with role-specific, customizable dashboards
- Protocol Status Tracking: Real-time visibility into protocol status and pending actions
- Ad-hoc Notifications: Send targeted communications to relevant designated users and stakeholders
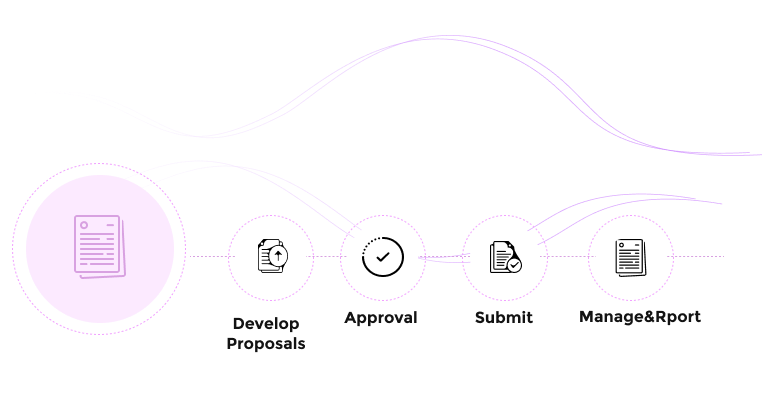
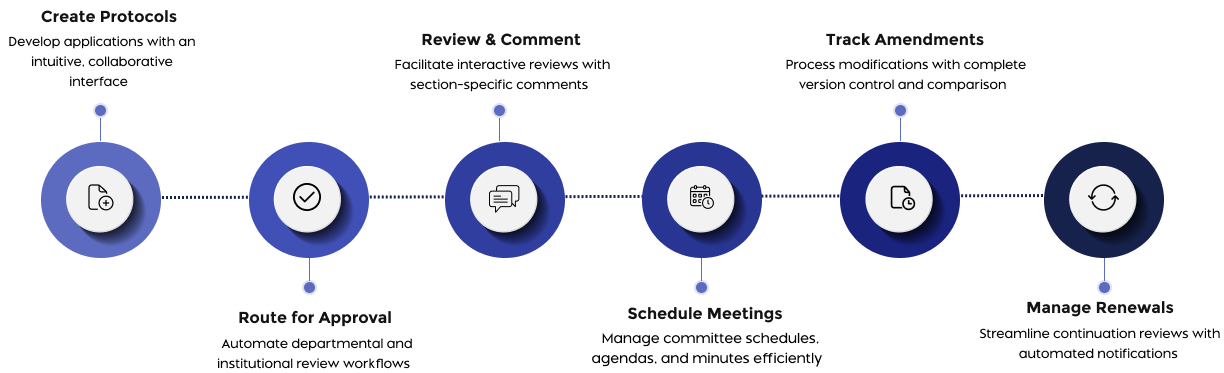
How Fibi IRB Works
FAQs
What is Fibi IRB and what does it do?
Fibi IRB is an electronic IRB (Institutional Review Board) management system that streamlines human subjects research compliance. It provides a unified platform for all IRB activities: protocol submissions, reviews, committee management, and reporting. By digitizing these processes, Fibi IRB helps institutions enhance transparency, ensure regulatory adherence, and speed up approvals.
How does Fibi support the IRB protocol lifecycle?
Can Fibi IRB handle meetings and reviews?
Is Fibi IRB customizable to our institution’s workflow?
Does Fibi IRB help ensure compliance and tracking?
Can Fibi IRB integrate with other Fibi modules?
Can Fibi IRB be deployed on-premise or in the cloud?
What is the typical implementation timeline for Fibi?
What kind of training and support is included in the implementation process?
Can institutions migrate legacy data into Fibi during implementation?
The Complete
Fibi Research Management Suite
Fibi IRB integrates seamlessly with the full Fibi research management suite—bringing together compliance, pre-award, and post-award activities in one connected platform:
Ready to Transform Your IRB Process?
Experience how Fibi IRB can revolutionize research compliance.



